SCCS Clears Micron‑Sized Silver for Cosmetics at Defined Use Levels
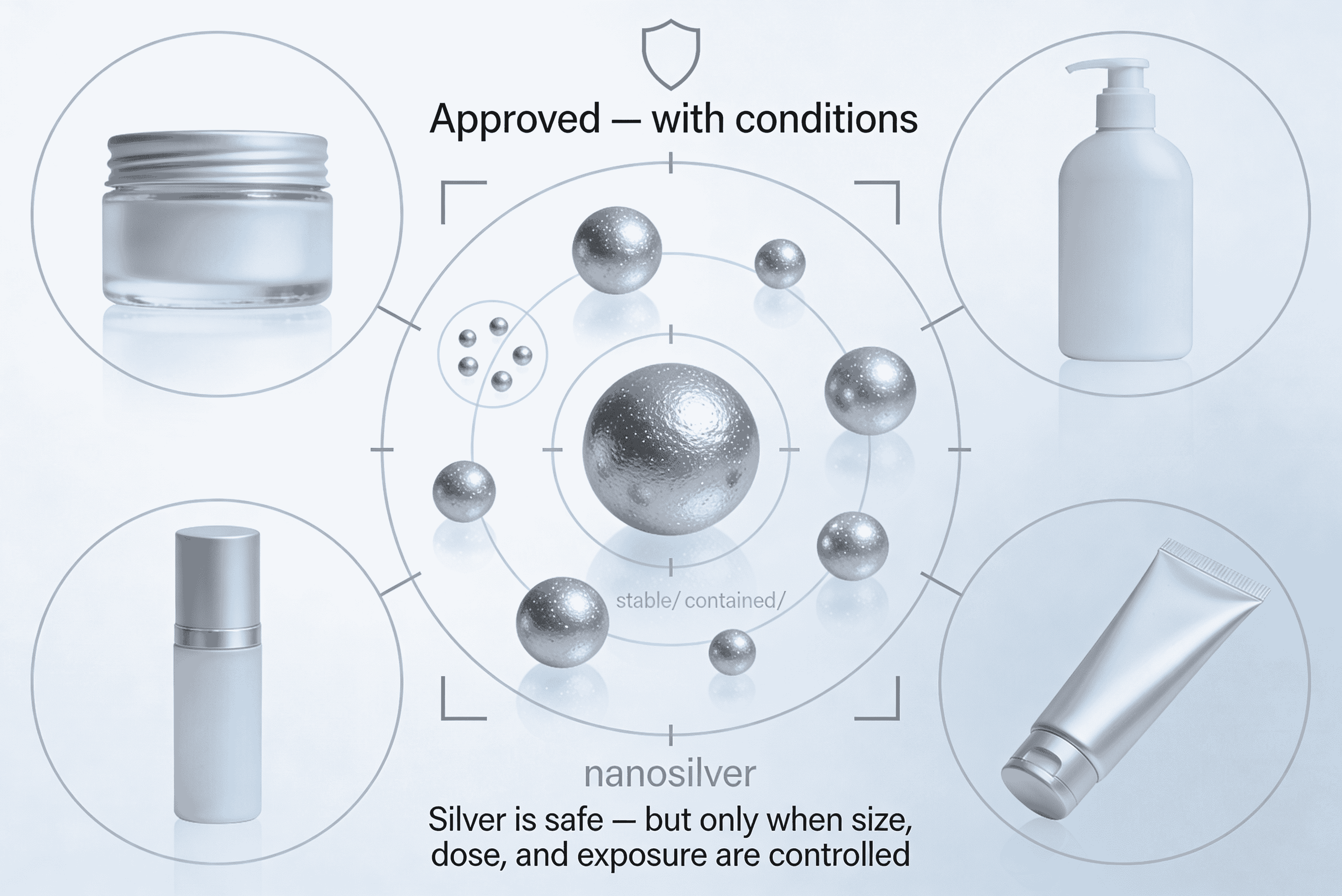
The SCCS has confirmed that micron‑sized silver can be considered safe in cosmetic products, but only at *defined use levels* and under specific conditions of application.
– The Scientific Committee on Consumer Safety (SCCS) reviewed silver (CAS No. 7440‑22‑4) in its micron‑sized form for cosmetic use.
– Their final opinion (July 2024) states that silver is safe when used in cosmetics within the concentration limits and product categories assessed.
– The SCCS emphasized that safety depends on controlled exposure levels and that silver should not accumulate in the body beyond acceptable thresholds.
Key Considerations
– Particle size matters: The SCCS specifically evaluated *micron‑sized* silver, not nanosilver. Nanoparticles may behave differently and raise separate safety concerns.
– Defined use levels: The committee set maximum concentrations for certain product types (e.g., leave‑on vs rinse‑off), ensuring consumer exposure remains below toxicological limits.
– Toxicological endpoints: The review considered systemic absorption, local irritation, sensitization, and long‑term accumulation risks.
Comparison: Micron‑Sized Silver vs Nanosilver
Aspect Micron‑Sized Silver (SCCS Opinion) Nanosilver (Previous SCCS Opinions)
Particle size Micron scale (>100 nm) Nano scale (<100 nm)
Safety conclusion Safe at defined use levels Concerns remain, not fully cleared
Absorption potential Lower systemic absorption Higher due to small size
Regulatory stance Approved with restrictions More cautious, pending further data
Risks & Limitations
– Overuse or higher concentrations could lead to silver accumulation in tissues, raising risks of argyria (skin discoloration) or systemic toxicity.
– Formulation context matters: Safety depends on product type (e.g., creams vs sprays) and frequency of use.
– Nanoparticle confusion: Brands must clearly distinguish micron‑sized silver from nanosilver in claims and labeling.
Why This Matters for the Beauty Industry
For formulators and marketers:
– Micron‑sized silver is now validated as a safe antimicrobial/functional ingredient in cosmetics, opening opportunities for innovation in skin care and hygiene products.
– Clear regulatory backing helps brands position silver‑based products confidently, provided they adhere to SCCS‑defined limits.
– Transparency in communication is key—highlighting compliance with SCCS safety levels reassures consumers and regulators alike.
Approved Concentration Limits
– Leave‑on products (face creams, lotions, etc.) – Safe up to 0.2% micron‑sized silver.
– Rinse‑off products (shampoos, shower gels, etc.) – Safe up to 1.0% micron‑sized silver.
– Oral hygiene products (toothpaste, mouthwash) -Safe up to 0.2% micron‑sized silver.
– Deodorants/antiperspirants – Safe up to 0.2% micron‑sized silver.
Note
– These limits apply only to micron‑sized silver (not nanosilver).
– The committee stressed that aggregate exposure across multiple products must remain below toxicological thresholds.
– Silver should not be used in ways that increase systemic absorption (e.g., inhalable sprays).
– The opinion is based on current toxicological data; future findings could adjust these limits.
Quick Reference Table
Product Type Max Safe Concentration
Leave‑on cosmetics 0.2%
Rinse‑off cosmetics 1.0%
Oral hygiene products 0.2%
Deodorants 0.2%
Source: SCCS Final Opinion on Silver (micron‑sized), July 2024
Subscribe to our free newsletter to read the latest news and articles before they are published.









Subscribe To Our Newsletter
Join our mailing list to receive the latest news and updates from The Cosmetics industry
You have Successfully Subscribed!